21 CPD Hours
Accredited by DHA

22 CPD credits
Accredited by SHPA

22 CME credits
Accredited by ICHS

About DUPHAT
MENA’s largest gathering of the entire pharmaceutical value chain – from suppliers, manufacturers, distributors to pharmacists.

95
Countries

150
International Speakers
Why Exhibit?
DUPHAT is committed to providing a complete solution to the pharmaceutical industry. This unique event brings the entire value chain of the pharma industry from the suppliers of raw material to the manufacturers of formulation, to the distributors and pharmacy networks.
- Build relations and network with 26,400+ Attendees
- Pre-book B2B meetings with 60+ handpicked Buyers
- Generate new leads from 75+ Participating Countries
Be part of the largest pharma business networking event attracting buyers & Suppliers from across the MEA region

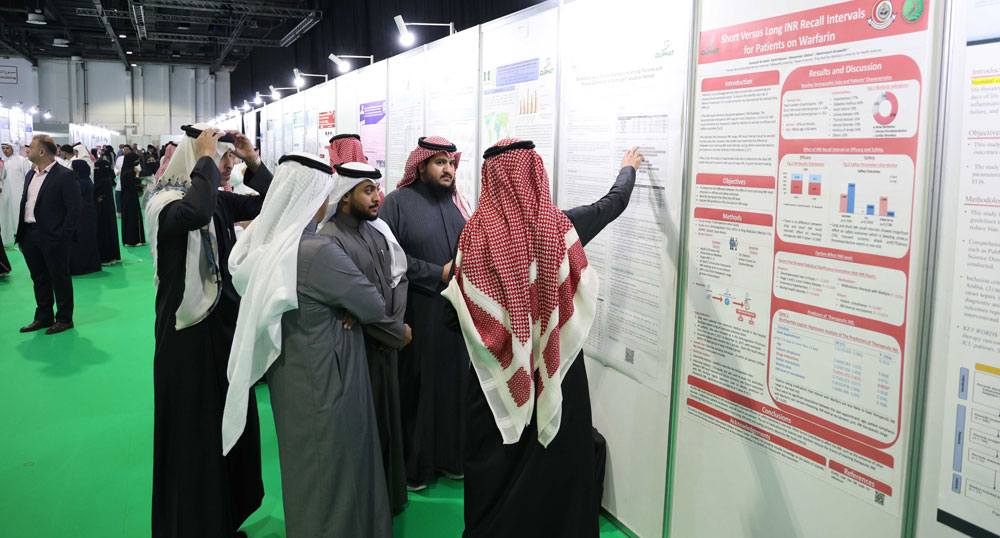
I've been attending DUPHAT for 20 years, seeing remarkable growth, especially this year post-pandemic. It's a vital platform for distributors like us to showcase services and connect with suppliers. The healthcare and pharmaceutical sectors in the region hold significant importance with consistent growth expected. The event remains a prime networking opportunity. Join us next year to witness the industry's vibrancy firsthand.
Mr. Stephan Stauffer
General Manager, Arabian Ethicals, United Arab Emirates

Recently we came across DUPHAT and realized that this is a prime opportunity to at least get to know Middle East markets. We're also partly inspired because we are distributing a couple of products in Kuwait currently. So we saw this as just an example of an extra opportunity to maybe expand horizons in the Middle East. And we're very impressed so far, especially from the organizational standpoint of this show. So we've come from Poland to look for distributors here locally in the region, in the Middle Eastern region, so that they can distribute the products which we have already tried and tested in Poland and bring them here into the Middle East.
Adam Swiderski
Vice President, Farmina SP. Z.O.O, Turkey

DUPHAT is an international pharmaceutical conference and gathering of many international and regional pharmaceutical companies. So it is very important for Pioneer as an important company in the Iraqi market and also recently we export many products for Emirate and also Oman and Gulf area and we are looking to expand our exportation and regional market. So it is essential to be here. The pharmaceutical industry in the region, especially in the Gulf region and also the Middle East, is very growing nowadays. So it is the future for the pharmaceutical industry is very huge and promising as well.
Dr. Ali Jabbar
Sales & Marketing Manager, Pioneer Co. For Pharmaceutical industries, Iraq

First of all, thanks to DUPHAT for hosting us here. The reason why we came here is because we want to expand in the GCC and we want people to see our factory and to see what we're doing. And this is the best hub for pharmaceuticals, I think. And hopefully, we'll be expanding into the GCC soon. The diversity is all over here in this exhibition and people are coming from all over the world. to visit us and get to know us. So it's the UAE and DUPHAT so there's nothing to explain more. It's the best hub for the pharmaceutical industry right now in the Middle East.
Mr. WAEL. L Mirza
Board Member, Iraqi Pharmaceutical Industry, Iraq

We are a pharmaceutical company which mainly produces products based on herbal materials. We have our own factory and we also have a quality control laboratory. We're looking for local and international distributors interested in our products and that's why we are here today. The pharmaceutical industry is a very fast-growing industry nowadays and people prefer to buy products that are mainly based on herbal materials nowadays than chemical ones. So that's why we're here: we have herbal analogise of the chemical products.
Ms. Mariam Kveslava
Export Manager – NeoPharm, Georgia
Our Scientific Activities
Bringing the pharma industry together
Hear directly from world’s pharma experts & leaders at this 3 day pharmaceutical conference

An exclusive pharmaceutical exhibition for suppliers, manufacturers and distributors. Your connection to the pharma industry
Hand picked buyers for you to meet F2F at DUPHAT
A hot spot for Researchers to present your work before peers

Pharma Networking
Featured Speakers
Expert Speaker lineup
Over the course of three days, the DUPHAT conference brings the world’s most renowned, scientific speakers and inspirational business leaders.

Prof. Richard Segal, RPh., PhD,
Professor and Interim Chair, Director of Graduate Studies, University of Florida College of Pharmacy, | USA

Prof. Richard Segal, RPh., PhD,
Professor and Interim Chair, Director of Graduate Studies, University of Florida College of Pharmacy,
Professor and Interim Chair, Director of Graduate Studies, University of Florida College of Pharmacy,
Lectures by this speaker
Thursday, 2024-01-11
Pharmaceutical Outcomes and Policy
09:45 - 10:30
Wednesday, 2024-01-10
Therapeutic Outcomes Monitoring (TOM) and Medication Therapy Management Interventions
09:30 - 10:30

Dr. Ibrahim Al-Khars, MS, MBA,
General Manager, Governance, Compliance, and Investor Relations, Innovest Company. Co-founder & Head of The Scientific Board, Afaq Healthcare Education | Saudi Arabia

Dr. Ibrahim Al-Khars, MS, MBA,
General Manager, Governance, Compliance, and Investor Relations, Innovest Company. Co-founder & Head of The Scientific Board, Afaq Healthcare Education
Dr. Ibrahim Al-Khars is the Co-Founder & Member of the Scientific Committee of Afaq Healthcare Training & Consultation Center and the General Manager, Governance, Compliance, and Investors Relations for Innovest Company, Saudi Arabia. Dr. Al-Khars has a Master in Applied Business Research from Swiss Business School, Switzerland; a Master in Business Administration specialized in International Business from the University of Florida, USA and a special diploma degree in new venture creation from the same university. He received a Bachelor degree in Pharmaceutical Science from King Saud University, Riyadh, Saudi Arabia with honor and Master Degree in Clinical Pharmacy from the same university. His academic history includes a Specialty Residency in Pharmacy Practice Management at Detroit Receiving Hospital and Wayne State University, Detroit, Michigan, USA. His research interests include health care quality improvement, corporate governance, health care reform, health care educational system and technologies, and pharmacy practice management. In addition to his teaching and management activities, Dr. Al-Khars has conducted many presentations, executive seminars and management development programs in different countries including Saudi Arabia, USA, Germany, Malaysia, Lebanon, Oman, Egypt, and United Arab Emirates.
Sessions by this speaker
Wednesday, 2024-01-10
PERSPECTIVES IN PHARMA
09:00 - 10:30
Lectures by this speaker
Wednesday, 2024-01-10
Rise of The Machine: Artificial Intelligence for Maximum Pharmacist Impact
09:00 - 09:45
Thursday, 2024-01-11
Going Below the Surface: Ethics in Pharmacy Practice
10:00 - 11:00

Prof. Albert Wertheimer, PhD, MBA
Nova Southeastern University, Florida, | USA

Prof. Albert Wertheimer, PhD, MBA
Nova Southeastern University, Florida,
Nova Southeastern University, Florida, USA
Sessions by this speaker
Wednesday, 2024-01-10
DRUG PRICING AND NEW STRATEGIES
09:00 - 10:30
Lectures by this speaker
Wednesday, 2024-01-10
Potential strategies to get needed new antibiotics
09:45 - 10:30
Thursday, 2024-01-11
Potential strategies to produce new antibiotics
10:00 - 11:00

Dr. Sally Ridgers,
Head of Education, Society of Hospital Pharmacists of Australia, SHPA, | Australia

Dr. Sally Ridgers,
Head of Education, Society of Hospital Pharmacists of Australia, SHPA,
Head of Education, Society of Hospital Pharmacists of Australia, SHPA, Australia
Lectures by this speaker
Tuesday, 2024-01-09
Advancing Practice 2.0: developing an updated practice recognition program.
11:00 - 11:45

Prof. Reza Karimi, RPh, PhD,
Professor, Pacific University School of Pharmacy, Oregon, USA | USA

Prof. Reza Karimi, RPh, PhD,
Professor, Pacific University School of Pharmacy, Oregon, USA
Professor, Pacific University School of Pharmacy, Oregon, USA
Lectures by this speaker
Tuesday, 2024-01-09
The Roles and Impacts of Hormones on the Anabolism and Catabolism of Macronutrients During Daily Fasting
11:00 - 11:45
Wednesday, 2024-01-10
How Hormonal Release and the Anabolism and Catabolism of Macronutrients Assist Us in Extending Survival Time During Starvation
14:00 - 14:55

Prof. Ahmed A. Elmarakby, PhD
USA Board Certified Pharmacist, Professor of Pharmacology & Toxicology, Augusta University, Georgia, | USA

Prof. Ahmed A. Elmarakby, PhD
USA Board Certified Pharmacist, Professor of Pharmacology & Toxicology, Augusta University, Georgia,
USA Board Certified Pharmacist, Professor of Pharmacology & Toxicology, Augusta University, Georgia, USA
Lectures by this speaker
Tuesday, 2024-01-09
Future Role of Artificial Intelligence in Improving Pharmacy Practice
11:45 - 12:30

Prof. Abdulla Molokhia, PhD.,
Professor, Pharmaceutics, Former Chairman, National Organization for Drug Control, | Egypt

Prof. Abdulla Molokhia, PhD.,
Professor, Pharmaceutics, Former Chairman, National Organization for Drug Control,
Professor, Pharmaceutics, Former Chairman, National Organization for Drug Control, Egypt
Sessions by this speaker
Thursday, 2024-01-11
ISSUES IMPACTING THE INDUSTRY
09:00 - 10:30
Lectures by this speaker
Thursday, 2024-01-11
Leading Issues Currently Impacting the Pharmaceutical Industry
09:00 - 09:45

Prof. Emad Elazazy, PhD, PharmD,
Professor of Governance and Pharmaceutical Practices, Egypt | Egypt

Prof. Emad Elazazy, PhD, PharmD,
Professor of Governance and Pharmaceutical Practices, Egypt
Professor of Governance and Pharmaceutical Practices, Egypt
Lectures by this speaker
Thursday, 2024-01-11
Get in the Game; Artificial Intelligence in Pharmacy
12:15 - 13:00

Prof. Amany Tawfik, MD, MSc.,
Associate Prof. Eye Research Institute, Oakland University, William Beaumont School of Medicine, Eye Research Center (OUWB)/ERC, Rochester, | USA

Prof. Amany Tawfik, MD, MSc.,
Associate Prof. Eye Research Institute, Oakland University, William Beaumont School of Medicine, Eye Research Center (OUWB)/ERC, Rochester,
Associate Prof. Eye Research Institute, Oakland University, William Beaumont School of Medicine, Eye Research Center (OUWB)/ERC, Rochester,
Sessions by this speaker
Wednesday, 2024-01-10
NEW DRUGS, ACCESS, AFFORDABILITY & ACCOUNTABILITY
10:45 - 13:00
Lectures by this speaker
Wednesday, 2024-01-10
Retinal Diseases and Vitamins
10:45 - 11:30

Prof. Matthew M. Murawski, R.Ph., Ph.D.,
Associate Professor of Pharmacy Administration, Department of Pharmacy Practice, Purdue University, | USA

Prof. Matthew M. Murawski, R.Ph., Ph.D.,
Associate Professor of Pharmacy Administration, Department of Pharmacy Practice, Purdue University,
Associate Professor of Pharmacy Administration, Department of Pharmacy Practice, Purdue University,
Lectures by this speaker
Tuesday, 2024-01-09
Introduction of an Abstract, Representational Model of The Practice of Pharmacy
12:30 - 13:15
Wednesday, 2024-01-10
A Demonstration of ADDRESS™ (the ADverse DRug Event Screening System) and Analysis of Results of 2,157 Patient Screens
14:00 - 15:00
Growth Hormone Deficiency in the MENA Region
December 20, 2023
The right to good nutrition is out of reach for over 16 million children under 5 years old in the MENA region. Despite some improvements, trends in children’s nutrition have stagnated or worsened since 2000.
Helicobacter Pylori: A brewing threat in the Middle East and North Africa
December 6, 2023
Gastric mucosa-associated lymphoid tissue (MALT) lymphoma, gastric cancer, and peptic ulcers are serious gastroduodenal diseases that can develop from a stomach infection known as Helicobacter pylori (H. pylori).

Opening our eyes to the burden of Uveitis
December 6, 2023
Uveitis is one of the most common ophthalmological conditions and is associated with visual impairment and decreased quality of life worldwide. It is a form of inflammation in the eyes that can affect the uvea, the middle layer of the eyeball, which includes the iris, ciliary body, and choroid.

Advancing Parkinson’s Disease Care in MENA Region: Unmet Needs and Current Progress
October 13, 2023
Major Depressive Disorder (MDD) is a significant medical condition that profoundly influences an individual’s mood, cognitive faculties, and behavior while negatively affecting their physical health.

MDD in MENA: Navigating Cultural Complexities in Mental Health
October 11, 2023
Major Depressive Disorder (MDD) is a significant medical condition that profoundly influences an individual’s mood, cognitive faculties, and behavior while negatively affecting their physical health.
Partners & Sponsors
DUPHAT SUPPORTERS
Special thanks to the support, involvement and active contribution of our Sponsors and Partners.