DATE
May 23, 2024
CATEGORY
Blog
SHARE

Introduction
The Middle East, a region rich in history and culture, is also witnessing significant strides in healthcare, particularly in the fields of vaccines and regenerative medicines. As the world grapples with various health challenges, including infectious diseases and chronic conditions, the Middle East is actively embracing innovative solutions to enhance public health outcomes.
Vaccination programs play a crucial role in preventing infectious diseases and safeguarding public health. In recent years, the Middle East has made notable progress in vaccine development, distribution, and uptake. Governments and healthcare authorities in the region have prioritized immunization campaigns to combat communicable diseases such as measles, polio, and influenza.
Prior to COVID-19, the MENA region presented a high coverage (90%) for routine childhood vaccinations, indicating a historically high acceptance of vaccination. This trend reversed during the pandemic, as research showed that the region had one of the lowest COVID-19 acceptance rates globally. A range of barriers amplify low vaccine acceptance, even when vaccines are available. According to the Behavioral Drivers Model (BDM), these barriers can be considered at three levels:
- Psychological and individual barriers
- Sociological barriers,
- Environmental and systems barriers (WHO, 2024)
Regenerative Medicine in the Middle East
The process of rejuvenating, replacing, regenerating, and restoring cells and establishing healthy function is called Regenerative Medicine (RM). There is already a strong RM market in the Middle East and further growth is expected. Healthcare cities and healthcare clinics offer a variety of RM options, especially for musculoskeletal ailments, cardiovascular diseases, and aesthetic or cosmetic dermatology. Stem cell therapy has grown in popularity in the UAE.
Certain trends in the healthcare sector of the Middle East favor the growth of the RM industry. One is that the Middle East is shifting from importing drugs and therapeutic products towards allocating funds for the local manufacture of pharmaceutical and therapeutic products.
Another trend is greater government investment in biomedical research and development. This encompasses advanced biotherapies such as cell therapy, immunomodulation therapy, gene therapies, and tissue engineering. These emerging medical technologies will further drive the future growth of the RM market in the Middle East.
The downside of the massive growth in the healthcare sector of the Middle East has been chronic staffing inadequacies such as the availability of trained healthcare professionals. Expats have a high turnover and attrition in the Middle East, returning to their respective countries once their contracts terminate. The leadership of the UAE has recently addressed this problem by facilitating “Golden visas” which enable highly skilled foreign talents to stay for 10 years (Bapat, 2022).
Biobanking in the UAE
Most of the biobanks in the UAE are either cord blood banks or adipose stromal cell banks. Cord blood banking has a long history in the UAE. The Dubai Cord Blood Research Center (DCRC) began in 2006 as the first public bank accepting cord blood donations in the UAE, although since 2018 they only offered private services. The first family cord blood bank to open a laboratory in the UAE was Cryo-Save Arabia, also launched in 2006, and now known as CellSave Arabia. Until this year, the majority of the cord blood banks marketing to parents in the UAE have been shipping their cord blood collections to laboratories in the United Kingdom or India for processing and storage.
A 2020 law from the UAE Ministry of Health which became effective in January 2022 requires banks that collect cord blood units in the UAE to set up a laboratory and storage facility inside the UAE8. Compliance with this law is not visible to consumers yet, because cord blood banks have been given extensions to operate if they can demonstrate that they are taking steps to establish a laboratory. The law requires that applicants for a license to bank cord blood in the UAE must put up a guarantee bond of AED 10 million. The law further specifies that within two years of commencing operations, the laboratory facility must be accredited by AABB or JACIE.
Clinics that use adipose stromal cells (ADSC) or stromal vascular fraction cells (SVFC) from autologous sources are allowed in the UAE. The prevalence of these clinics has driven the creation of biobanks for adipose cells. A 2020 law from the Government of Dubai spells out the regulations on autologous adipose cellular therapies. The regulations allow a variety of techniques for the isolation of adipose cells, provided that the cells are minimally manipulated and are not combined with other therapeutic products. Specifically, isolation methods can include mechanical dissociation by liposuction, the use of enzymes (i.e., GMP collagenase is approved for use in the UAE), rapid sonication (sonication-mediated cavitation), and possibly other “FDA techniques” being developed (Bapat, 2022).
Regulations on the Practice of Regenerative Medicine in the UAE
Biotherapies can be approved for use in the UAE if they are approved by recognized regulatory bodies elsewhere, such as the United States FDA or an equivalent in another country, and also include international medical associations and professional bodies. The level of previous approval that will be considered is flexible, covering both biotherapies that are considered standard care for that indication, as well as biotherapies that are in officially registered clinical trials for that indication. Applications for approval of biotherapy in the UAE must include all the processes and protocols governing the production or manufacturing of the cells or their derivatives and their therapeutic uses.
RM therapies can only be provided in the UAE by licensed and authorized healthcare facilities. To be licensed to provide therapies with cells or cell derivatives, the healthcare facility must show evidence of Good Laboratory Practice (GLP), Good Medical Practice (GMP), Good Tissue Practice (GTP), and Good Clinical Practice (GCP) when applicable. The facility should be able to comply with and provide evidence of accreditation and international approvals applicable to the scope of services offered.
The UAE also has training requirements for the physician or surgeon that administers the biotherapy. The provider must present evidence of recognized training in RM with a clear description of the amount and the nature of the hands-on training received. This should include donor selection, patient eligibility and suitability, selection of procedures, preparation of patients, screening for infectious diseases, intraoperative and post-operative care, and follow-up. The provider should also be able to furnish records of demonstrated competency such as the number of cases treated and their outcomes, evidence of Continuing Medical Education credits, and the provider must hold an Advanced Cardiac Life Support (ACLS) or Advanced Life Support (ALS) certification where required (Bapat, 2022).
Vaccines in the Middle East
- The Middle East has made significant strides in vaccine utilization, with governments and healthcare authorities prioritizing immunization programs to prevent the spread of infectious diseases. Key vaccines, including those for influenza, polio, measles, and hepatitis, are widely available and administered through national vaccination campaigns and routine immunization schedules.
- In recent years, the introduction of novel vaccines has further enhanced disease prevention efforts in the region. Notably, the rollout of COVID-19 vaccines has been a major focus, with Middle Eastern countries actively participating in global vaccination initiatives to curb the spread of the virus. Governments have implemented comprehensive vaccination strategies, including mass vaccination centers, mobile clinics, and digital registration systems, to ensure widespread coverage and equitable access to vaccines.
- Despite progress, challenges persist in vaccine distribution and uptake, including vaccine hesitancy, logistical barriers, and disparities in healthcare infrastructure. Addressing these challenges requires collaborative efforts between governments, healthcare providers, and community stakeholders to promote vaccine literacy, strengthen healthcare systems, and improve access to immunization services.
- Countries across the Middle East are adopting a range of strategies to support vaccine rollout plans, which include brokering advance purchase agreements (APAs) directly with international pharmaceutical companies, leveraging their established international relations, and developing new ties with host governments of major vaccine suppliers, building up their own national vaccine production and distribution hubs, and participating in global vaccine development and distribution initiatives. Some countries are faring better than others and the prospects for the mass rollout of Covid‑19 vaccination varies considerably across the region (Farha et al., 2021).
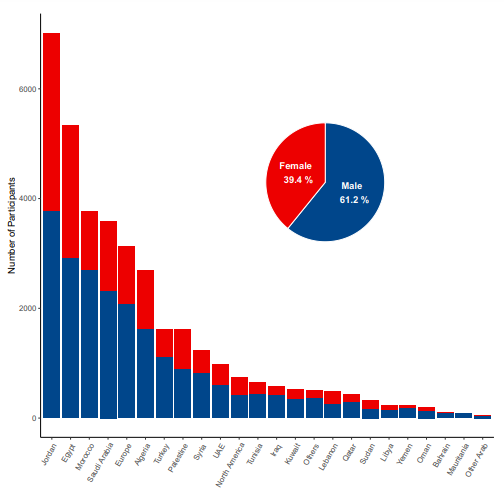
Source: (Qunaibi et al., 2021)
A bar plot and a pie chart showing the distribution of participants according to country of residence and gender
Participation in clinical trials
- Several countries across the Middle East and North Africa have participated in clinical trials for COVID-19 vaccines, which have helped them secure early and preferential access to vaccine shipments. Bahrain, Egypt, Jordan, Morocco, the UAE, Saudi Arabia, and Turkey have participated and continue to participate in trials of vaccines being developed by the Chinese pharmaceutical companies Sinopharm, Sinovac, and CanSino. The UAE completed stage three clinical trials of the Sinopharm vaccine and granted emergency use authorization for free-of-charge access to the entire population in December (earlier approval for frontline medical staff was granted in September). In addition, the UAE has participated in clinical trials of Sputnik V, a vaccine being developed by the Russian Gamaleya National Centre of Epidemiology and Microbiology (and marketed by the Russian Direct Investment Fund), while Turkey has participated in clinical trials of the vaccine developed by Pfizer-BioNTech (EIU, 2024).
Source (Kaadan et al. 2021)
Emerging storage and distribution hubs
- Strategic importance as storage and distribution hubs could benefit some countries in securing access to vaccines and implementing their national rollout plans. The UAE, Egypt, and Israel have the potential to emerge as key logistics centers that facilitate the storage and distribution of coronavirus vaccines across the Middle East and North Africa.
- The UAE intends to leverage its position as a global logistics hub with technically advanced transport and storage facilities to facilitate the distribution of COVID-19 vaccines throughout the region and the wider world. The Hope Consortium has been set up involving Etihad Cargo, Abu Dhabi Ports, and existing global partners to transport 6bn doses of approved vaccines around the world in cold and ultra-cold storage facilities during the first half of 2021, rising to 18bn doses transported by the end of the year. The consortium has strong expertise in pharmaceutical logistics, specialist pharmaceutical, and healthcare services, and a myriad of existing regional and global supply routes that put it in a strong position to achieve its delivery targets assuming delivery of already approved vaccines.
- Egypt has been identified as a potential storage and distribution hub owing to its strategic location, strong logistics offering, and local manufacturing capabilities in the pharmaceutical sector. In addition, Israel could emerge as a regional distribution hub with the government pushing hard to develop logistics capabilities that will support national vaccination rollout efforts and at the same time contribute to the global vaccine supply chain (EIU, 2024).
Source: Statista 2024
Challenges and Opportunities:
- While the Middle East is witnessing rapid advancements in vaccine and regenerative medicine utilization, several challenges need to be addressed to realize the full potential of these interventions. These challenges include regulatory frameworks, ethical considerations, infrastructure limitations, and workforce capacity.
- Regulatory frameworks governing the approval and oversight of vaccines and regenerative therapies vary across Middle Eastern countries, posing challenges for cross-border collaboration and technology transfer. Harmonizing regulatory standards and promoting regulatory convergence can facilitate the timely approval and adoption of innovative medical interventions.
- Ethical considerations surrounding the use of vaccines and regenerative medicines, including issues related to patient consent, privacy, and equity, require careful deliberation and adherence to international ethical guidelines. Middle Eastern countries can benefit from engaging in dialogue with global stakeholders and fostering ethical frameworks that prioritize patient welfare and societal values.
- Infrastructure limitations, particularly in rural and underserved areas, pose barriers to the delivery of vaccines and regenerative therapies, hindering access for vulnerable populations. Investments in healthcare infrastructure, including the expansion of healthcare facilities, deployment of telemedicine solutions, and training of healthcare personnel, are essential to address these disparities and ensure equitable access to healthcare services.
- Furthermore, building workforce capacity in vaccine development, regenerative medicine research, and clinical practice is crucial for sustaining healthcare innovation in the Middle East. Educational programs, professional training initiatives, and collaborations with international experts can enhance the skills and expertise of healthcare professionals and scientists in the region.
- Despite these challenges, the Middle East is poised to capitalize on the opportunities presented by vaccines and regenerative medicines to improve public health and advance medical science. By fostering collaboration, innovation, and investment in healthcare infrastructure and human capital, Middle Eastern countries can strengthen their healthcare systems and contribute to global efforts to address pressing health challenges.
Conclusion
The Middle East is witnessing a transformative era in healthcare, marked by advancements in vaccines and regenerative medicines that hold immense promise for disease prevention, treatment, and personalized medicine. While challenges remain, the region is well-positioned to overcome barriers and leverage its scientific and technological capabilities to improve health outcomes and enhance the quality of life for its inhabitants. Through strategic investments, partnerships, and policy reforms, the Middle East can emerge as a leader in healthcare innovation and contribute to global health security and prosperity.
Nevertheless, the Middle East is poised to harness the full potential of vaccines and regenerative medicines through strategic investments in healthcare infrastructure, workforce development, and cross-sector partnerships. By embracing a holistic approach that prioritizes innovation, equity, and patient-centered care, the region can emerge as a global leader in healthcare innovation, driving positive health outcomes and advancing the well-being of its diverse population.
Source: Delveinsight http://www.delveinsight.com/






